electron configuration xe|Iba pa : Manila The ground-state electron configuration of xenon is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6. This electron configuration shows that the last shell of xenon has eight electrons. Therefore, the valence electronsof xenon are eight. The elements in group-18 of the . Tingnan ang higit pa 13,688 pinay sa kotse FREE videos found on XVIDEOS for this search. Language: Your location: USA Straight. Login Join for FREE Premium. Best Videos; Categories. Porn in your language; . Gustong labasan ni GF kaya sinakyan ako kantutan sa kwarto 6 min. 6 min Pinay Cowgirl69 - 1.7M Views - 720p. Tinira sa pwet ng mabilisan si pinay 8 min. 8 .
PH0 · writing electron configurations for ions
PH1 · shorthand electron configuration
PH2 · full electronic configuration for lead
PH3 · electron configuration guide
PH4 · electron configuration for every element
PH5 · electron configuration chart
PH6 · arsenic electron configuration
PH7 · abbreviated electron configuration for cs
PH8 · Iba pa
As determined by IMDb users. Our Most Popular charts use data from the search behavior of IMDb's more than 250 million monthly unique visitors to rank the hottest, most buzzed about movies and TV shows.
electron configuration xe*******The ground-state electron configuration of xenon is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6. This electron configuration shows that the last shell of xenon has eight electrons. Therefore, the valence electronsof xenon are eight. The elements in group-18 of the . Tingnan ang higit pa
The total number of electrons in xenon is fifty-four. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in xenon in . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa
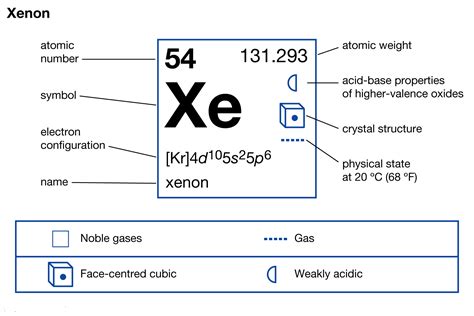
Mar 23, 2023 These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Atomic numberThe number of protons in an atom. .Xenon has atomic number 54; that is, its nucleus contains 54 protons. At standard temperature and pressure, pure xenon gas has a density of 5.894 kg/m , about 4.5 times the density of the Earth's atmosphere at sea level, 1.217 kg/m . As a liquid, xenon has a density of up to 3.100 g/mL, with the density maximum occurring at the triple point. Liquid xenon has a high polarizability due to its lar.Full electron configuration of xenon: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6. iodine ← xenon → cesium. Xenon, complete electron configuration.
Xe, 54, xenon : 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 Rn, 86, radon : 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 Og, 118, . Xenon Electron Configuration (Xe) with Orbital Diagram. August 10, 2021 Leave a Comment. Check out the Xenon electron configuration here and get the proper .
The shorthand electron configuration of Xe is [Kr] 4d105s25p6, where [Kr] is the noble gas in the row above xenon. This is the base that we use to form the configuration. The web page explains .
The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. By knowing the electron configuration of an element, we can predict and .
3.1: Electron Configurations. Page ID. Skills to Develop. Derive the predicted ground-state electron configurations of atoms. Identify and explain exceptions to predicted electron .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) . Xe . The periodic table can be a powerful .
electron configuration xeThis page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.Xe (Xenon) is an element with position number 54 in the periodic table. Located in the V period. Melting point: -111.9 ℃. Density: 0.00449 g/cm 3 . Electronic configuration of the Xenon atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6Electronic configuration of the Xenon atom in ascending .Iba pa The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the .
electron configuration xe Iba paSummary. The Electron: Crash Course Chemistry #5. Video 2.6.2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on).
In several cases, the ground state electron configurations are different from those predicted by Figure 6.8.1 6.8. 1. Some of these anomalies occur as the 3 d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4 s1 3 d5 rather than the predicted [Ar]4 s2 3 d4. The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and . The electronic configuration of Xenon is [Kr] 4d10 5s2 5p6. The step-by-step approach to writing the electronic configuration as per Aufbau principle is as follows: The first two electrons enter the 1s orbital, which can have a maximum two electrons. Next, two electrons enter the 2s orbital, followed by 6 electrons in the 2p orbital.

An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled whenever possible. . Xe: 2: 2 6: 2 6 10: 2 6 10: 2 6 .Introduction to electron configurations. Google Classroom. About. Transcript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan. The electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the valence electrons (e.g. [Xe] 6s2 for barium). Oxidation States Oxidation states are typically represented by integers which may be positive, zero, or negative.The electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the valence electrons (e.g. [Xe] 6s2 for barium). Oxidation States Oxidation states are typically represented by integers which may be positive, zero, or negative.For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration . For example, cerium has an electron configuration of [Xe]6s 2 4f 1 5d 1, which is impossible to rationalize in simple terms. In most cases, however, these apparent anomalies do not have important chemical consequences. Table 1 summarizes .The atomic number of Xe is 54 and its electronic configuration is given by: 2, 8, 18, 18, 8. Suggest Corrections. 42. Similar questions. Q. Xenon has closed shell configuration but is known to give compounds with fluorine because: Q.
Electron configuration 4d 10 5s 2 5p 6: Electrons per shell: 2, 8, 18, 18, 8: . The first excimer laser used a xenon dimer (Xe 2) energized by a beam of electrons to produce stimulated emission at an ultraviolet wavelength of 176 nm. Xenon chloride and xenon fluoride have also been used in excimer (or, more accurately, exciplex) lasers. . This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait — you can avoid .
EWTN is a global, Catholic Television, Catholic Radio, and Catholic News Network that provides catholic programming and news coverage from around the world.
electron configuration xe|Iba pa